Geographical Distribution in Africa
Geographical Distribution of the Sweet potato weevils in Africa (red marked). Updated on 11 July 2019. Source CABI
General Information on Pest and Damage
Introduction
The African sweet potato weevil (Cylas puncticollis) is one of the most important pests of sweet potato in tropical Africa, notably Uganda, Rwanda, Kenya and Cameroon. Cylas brunneus is known from West and Central Africa and some countries in East Africa (Rwanda, Burundi and Kenya). These two species are found together attacking sweet potatoes in East and West Africa (Hill, 1983). Cylas formicarius is a destructive pest of sweet potato throughout most of the tropical and subtropical regions and occurs in several African countries.
Damage
Adult weevils feed on leaves, the underground storage roots (tubers) and the vines of sweet potatoes. They prefer to feed on storage roots, but at the beginning of the growing season, when the plants have not yet produced storage roots, the adult weevils live on the stem and leaves. They lay eggs on vines and leaves, and the grubs will feed in the stem or the leaf and pupate inside the vines.
As the plant gets older and starts to form storage roots, the weevils search for exposed roots. Since they cannot dig, they reach the tubers through cracks in the soil. Weevils feed on the storage roots and lay eggs just below the surface of the root. Feeding and egg-laying punctures (numerous small holes) lower the quality of the root and can reduce the market price. If roots with egg punctures are stored they will serve as source of infestation for the clean roots stored beside them. Adult feeding on the foliage seldom is of importance.
The grubs are more damaging, feeding, boring and making tunnels into the stems and roots. Damage to the stems may cause serious mortality to seedlings. Allard et al. (1991) reported on serious weevil attacks on sweet potato nurseries in Ethiopia. Feeding in the vines causes thickening and malformation and often cracking of the tissue. A damaged vine is discoloured, cracked, or wilted. Stem damage is believed to be the main reason for yield loss, although damage to the vascular system caused by feeding, larval tunnelling and secondary rots reduce the size and number of roots.
 |
| Sweet potato tuber showing damage of sweet potato weevil |
|
© Courtesy of Institute of Plant Biotechnology for developing Countries, Ghent University, Belgium
|
An infested tuber is often riddled with cavities or tunnels These wounds serve as entry point for infections, causing rotting of tubers. Attacked tubers start rotting from the top and develop an unpleasant smell and a bitter taste, making them unfit for human consumption. Even low level of infestation can reduce root quality and marketable yield because the plants produce a bitter toxin (terpenoid) in response to feeding by weevils.
Weevil damage increases the longer the crop remains not harvested. In Kenya, where farmers practice piece meal harvesting, losses are in the order of 10%. Pest damage usually continues during storage, therefore infested tubers cannot be stored for a long time. In conjunction with other beetle pests, C. puncticollis can completely destroy sweet potato plantations.
Damage by weevils can be recognised by the holes in the vines or the tunnels in the tuber when you pull them up from the soil.
Host range
The main host of all species of sweet potato weevil is sweet potato. Alternative hosts are morning glory, water spinach (Ipomoea aquatica) and other Ipomea weeds. Cylas bruneus has only been reported on sweet potatoes. Cylas puncticollis has also been reported on coffee, maize, cowpea, sesame, and Cassia acutifolia (C. senna) (CABI).
Symptoms
A symptom of infestation by sweet potato weevils is yellowing, cracking and wilting of the vines, but a heavy infestation is usually necessary before this is apparent. Damage by weevils can be recognised by the holes in the vines or the tunnels in the tuber when you pull them up from the soil. Attacked tubers become spongy, brownish to blackish in appearance.
Affected plant stages
Flowering stage, fruiting stage, post-harvest and vegetative growing stage.
Affected plant parts
Leaves, roots and stems.
Symptoms by affected plant part
Leaves: external feeding
Roots: rot; internal feeding; external feeding.
Stems: external discolouration; abnormal forms; internal feeding; external feeding.
Biology and Ecology of Sweet Potato Weevil
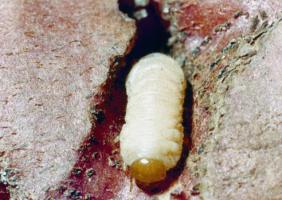 |
| Sweet potato weevil larvae on sweet potato. The full-grown larva is 7.5-8.0 mm long. |
|
© Clemson University - USDA Cooperative Extension Slide Series, Bugwood.org
|
The egg is oval in shape and yellowish-white in colour. It is laid singly in small cavities on the sweet potato root or at the base of the vine. The cavity is then sealed with a plug of the mother's excrement (faecal material). The egg hatches in about 3 to 7 days depending on the environmental conditions.
The larva is a legless grub, white in colour. The fully-grown grub is about 8 mm long. The head is comparatively large, and brown or pale-yellow. The body is slightly curved. The grub is found feeding on the vine near the base of the plant and goes down to the roots to feed. Larvae develop for 11 to 33 days before pupating.
The fully-grown grub turns into a pupa in an enlarged area of the feeding tunnel. The pupa is whitish and about 6 mm long. Initially it is white, but with time it becomes greyish in colour with darker eyes and legs. The pupa is similar to the adult in appearance, although the wings, the head and the long snout are bent downwards. Adults emerge after 7 to 28 days depending on the environmental conditions.
 |
| Sweet potato weevil. Adult female, body length 6-8 mm. |
|
© Courtesy EcoPort (http://www.ecoport.org): Land Care Ltd. New Zealand
|
The adult insect is a weevil. Weevils are beetles with a long pointed snout. The body of the sweet potato weevil is slender resembling ants. The length of the adult is between 6 to 8 mm. They vary in colour and in size according to the species. Cylas puncticollis is larger and entirely black. Cylas brunneus is brown with blue or bluish-green elytra (hard wings) and reddish legs, and is smaller than C. puncticollis. Cylas formicarius is as small as C. brunneus but has a bluish-black abdomen and a red thorax.
The weevils complete their lifecycle in the storage roots (tubers). They flying infrequently and generally only for short distances (500 to 1000 m). The development of the weevil from egg to adult takes 32 days in average.
Pest and Disease Management
Pest and disease management: General illustration of the concept of Infonet-biovision
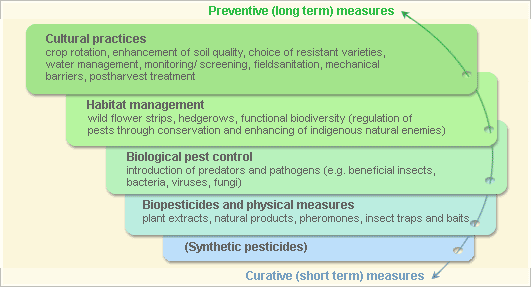
This illustration shows the methods promoted on infonet-biovision. The methods shown at the top have a long-term effect, while methods shown at the bottom have a short-term effect. In organic farming systems, methods with a long-term effect are the basis of crop production and should be of preference. On the other hand methods with a short-term effect should be used in emergencies only. On infonet we do not promote synthetic pesticides.
Further below you find concrete preventive and curative methods against Sweet potato weevils.
Cultural practices
Monitoring
At the beginning of the growing season, when the plants have not yet produced any storage roots, the adult weevils are commonly found on the foliage, but they quickly drop to the ground if disturbed. During the day they often hide under leaves or in soil cracks. Most of the larvae are found in the upper 15 cm of the tubers and basal 10 cm of the vine. Select storage roots that appear soft, smelling, or have external scarring or small, darkened holes. Cut these open and look for tunnelling and larvae. Pheromone traps are useful in monitoring weevil populations, but this technology is expensive and not widely available. Efforts are going on to develop pheromones traps for monitoring and control in East Africa (refer to section on traps below).
Among various control measures attempted, modification of cultural practices has the greatest potential in combating the sweet potato weevil at low cost.
Crop rotation
Avoid planting sweet potatoes in the same area for two to three successive seasons. It has been suggested that, if possible, sweet potatoes should be grown in a field only once every five years. Rice and sorghum are often used in rotation with sweet potatoes. This rotation will help break up the cycle of the weevil and will help to control sweet potato weevil, particularly if integrated with other management approaches, such as the ones described below.
Intercropping
Experimental studies in Taiwan showed that intercropping with chickpea, coriander, pumpkin, radish, fennel, black gram and yard long bean reduced weevil infestations considerably. However, intercropping with black gram, fennel, pumpkin, and yardlong bean also reduced sweet potato yields. The best results were obtained with coriander. Similarly, reduced weevil damage was observed when sweet potato was intercropped with proso millet and sesame, but sweet potato yield was also considerable reduced. Sweet potato has been found to inhibit germination of proso millet (Peterson et al., 1999).
Planting time
Plant early or plant early maturing varieties. This will allow harvesting before the end of the growing season, minimising the risk of drought and consequently the damaging effect of weevils, which enter the soil through cracks.
Use of clean cuttings
Carry-over of the weevils from an infested crop to the new planting could be reduced by carefully selecting fresh cuttings for planting a new crop. Use clean (insect-free) vines as planting material. Prefer planting material from vine tips. Weevils tend to lay eggs in the older woodier parts of the vine, so if the tender tips are used for planting they are less likely to be infested by weevils. Studies in Taiwan showed that cuttings (25 to 30 cm long) taken from fresh terminal growth, even from an infested crop, were rarely infested with weevils, whereas older portions of the stem were (AVRDC).
Keep distance to infested fields
Planting away from weevil-infested fields, and or use barrier crops such as cassava, maize, bananas or sorghum planted around the perimeters in stripes of at least 3 to 5 m in width between fields have been observed to restrict movement of weevils between fields (CIP, VITAA).
Avoid soil cracking
Avoid or minimise cracks in the soil. Soil cracks are the major route of weevil access to roots. This can be done by:
- Planting cuttings deep in the soil and using of deep-rooted cultivars reduce weevil damage. The growth of roots, especially in cultivars that set roots near the soil surface can produce cracks and increase exposure of roots to the weevil.
- Ridging: it prevents the soil from cracking by hilling the area around the plant. Re-hill mounds about 30 days after planting to close soil cracks. Close ridges after piece meal harvests to cover exposed tubers. This should be implemented before the adult weevil reaches the roots.
- Mulching: mulches conserve soil moisture and minimise soil cracking. The physical cover made by mulching materials further reduces access of roots to the weevil even if the soil cracks. The soil surface should be covered soon after planting and the cover should be maintained until harvest.
- Routine irrigation: it is important to provide sufficient water to prevent soil cracking. This is a practical method for farmers with a reliable water supply.
Sanitation
Remove and destroy (through burying, burning or feeding to livestock) any crop residues left in the field after harvest. Infested roots must be completely buried (over 15 cm deep); avoid cracks, which allow emerging weevils to reach the soil surface. If vines are left in the field to improve soil fertility, care should be taken to ensure they are dead and not able to sprout. Care should be taken to remove and destroy any infested roots when doing piecemeal harvest.
Field sanitation is important because weevils survive in roots and stems and infest succeeding or neighbouring sweet potato plantings. However, to effectively reduce weevil infestation it should be practised in a large area or community. Clean cultivation is particularly important where rotation is not possible, for example in areas where sweet potato is a staple food and is planted year round.
Flooding of fields
Flooding of infested fields for at least 48 hours after completing harvest drowns weevils and induces rotting of the leftover plant materials and thereby reduces weevil densities from one planting to the next. This is an option in areas where rotation is not possible.
Flooding of fields between two consecutive sweet potato crops may reduce the immediate source of weevils from the field.
Early harvesting
Harvest the crop as soon as it has developed roots of acceptable size.
Control of alternative hosts
Alternative hosts of the sweet potato weevil (e.g. morning glory, water spinach, wild Ipomoea etc) can shelter weevils between planting seasons and serve as a source of weevil infestation when a new crop of sweet potato is planted. Therefore, removal of these host plants growing in the vicinity of sweet potato plantings is recommended as a control measure. However, indiscriminate elimination of these plants is not recommended since it may also lead to undesirable ecological effects. To minimise this, all Ipomoea could be eliminated for one cropping season and allowed to grow in the subsequent seasons, once the area is free of the weevils.
Biological pest control
Natural enemies
Predatory ants, earwigs, spiders and ground beetles are important predators of the sweet potato weevil. Among those ants seem to be most important. In Cuba, two species of predatory ants, Pheidole megacephala and Tetramorium guineense, which are common inhabitants of banana plantations, are used for control of sweet potato weevils. These ants are encouraged in reservoir areas, such as patches of forest, where they are naturally abundant. Ants nests are moved by a simple method using rolled banana leaves as 'temporary nests' to transport the ants from their natural reservoir to sweet potato fields, or banana stems, baited with honey, are placed in the reservoir areas and, when covered by ants, transported to the sweet potato fields. The ants then prey upon sweet potato weevils and other insects. Setting up colonies in the field 30 days after planting with 60 to 110 nests/ha can keep weevil infestation at low levels (3 to 5%) (FFTC; Sheehy Skeffington, 2006).
Disease-causing microorganisms, especially the fungus Beauveria bassiana, have been observed to cause high mortality of sweet potato weevils in the field under conditions of high humidity and high insect density. This fungus is commercially available in some countries. It can be used for treating the planting material and the soil to reduce soil infestation.
Traps
Pheromone traps are widely used for monitoring the sweet potato weevils. However, these traps are expensive and not widely available. In 1995, a collaborative project of the National Agricultural Research Institutes, Natural Resources Institute (NRI), and the International Potato Centre (CIP) began developing pheromones for monitoring and controlling the African sweet potato weevils in East Africa. Pheromone compounds that proved effective in catching male weevils under field conditions in Uganda were identified, and several traps were tested. A five-litre plastic jerry can with rectangular openings of 11×5 and 6×5 cm filled with 0.5 l soapy water (1 g Omo/1 l water), with 0.1 mg lures to be replaced every 8 weeks is presently the most effective and robust trapping system. Experiments on the use of these traps for mass trapping of males for weevil control are going on (CIP; Smit et al., 1997).
Farmers solution
Farmers in Kilifi Kenya have found their own unique solution to the control of sweet potato weevil; They make use of the abundantly available insect repellant Lantana camara bushes in their area at planting time. When preparing planting ridges for sweet potatoes, fairly large amounts of lantana leaves/branches are incorporated into the ridges. This serves 2 purposes:
1) it adds organic matter and goos fertilizer to the soil and
2) it keeps the sweet potato weevils away.
This simple planting method makes the Kilifi farmers able to produce larger and better quality sweet potatoes than most of their neighbouring communities.
Information Source Links
- Allard G.B, Cock M.J.W and Rangi D.K (1991). Integrated control of arthropod pests of root crops, Final Report. Nairobi, Kenya: CAB International.
- CABI. (2005). Crop Protection Compendium, 2005 Edition. CAB International Publishing. Wallingford, UK. www.cabi.org
- CIP. What is damaging my sweetpotato? A field guide for farmers on pests and diseases of sweetpotato in sub-Saharan Africa, 2006 www.sweetpotatoknowledge.org
- CIP. Enhancing the Role of Small-Scale Sweetpotato Starch Enterprises in Sichuan, China. Program report 95-96. www.cipotato.org
- Chalfant R.B., Jansson R.K, Seal D.R. and Schalk J.M, (1990). Ecology and management of sweet potato insects. Annual Review of Entomology, 35:157-180.
- Daiber K.C. (1994). Injurious insects, spider mites and nematodes on sweet potatoes in southern Africa. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 101(5):550-557.
- Dunguni FFS (Farmer Field School), Kilifi, Kenya
- FFTC. A. Sustainable Pest Management Strategy for Sweet potato Weevil in Cuba: A Success Story. INIVAP. Food & Fertilizer Technology Center for the Asian and Pacific Region. By A. Lagnaoui. www.agnet.org
- Hill, D. (1983). Agricultural insect pests of the tropics and their control. 2nd edition. Cambridge University Press. ISBN: 0-521-24638-5.
- OISAT (Organisation for Non-Chemical Pest Management in the Tropics) www.oisat.org
- Peterson, J.K., Harrison, H.F. Jr. and Snook, M.E. (1999). Comparison of three parameters for estimation of allelopathic potential in sweet potato(Ipomoea batatas (L.) Lam.) germplasm. Allelopathy Journal 6: 201-208.
- Pfeiffer H.J. (1982). Sweet-potato improvement in Cameroon. Root crops in eastern Africa. Proceedings of a workshop held at Kigali, Rwanda, 23-27 November 1980, 33-38.
- Plant Protection and Regulatory Services Directorate (PPRSD). (2000). Handbook of crop protection recommendations in Ghana: An IPM approach. Vol. 3: Root and Tuber Crops, Plantains. E. Blay, A. R. Cudjoe and M. Braun (editors). Published by PPRSD with support of the German Development Cooperation (GTZ). ISBN: 9988-8025-6-0.
- Sheehy Skeffington, M. (2006). Organic fruit and vegetable growing as a national policy: the Cuban story. www.energybulletin.net
- Smit N.E.J.M. and Matengo L.O., 1995. Farmers' cultural practices and their effects on pest control in sweetpotato in South Nyanza, Kenya. International Journal of Pest Management, 41(1):2-7.
- Smit, N. E. J. M.; Downham, M.C.A.; Odongo, B.; Hall, D.R. and Laboke, P.O. (1997). Development of pheromone traps for control and monitoring of sweetpotato weevils, Cylas puncticollis and C. brunneus, in Uganda. Entomologia Experimentalis et Applicata. Publisher Springer Netherlands ISSN0013-8703 (Print) 1570-7458 (Online) Issue Volume 85, Number 2 / November, 1997
- Talekar, N. S. (1991). Integrated control of Cylas formicarius. In Sweet potato Pest management: A global prespertive. Jansson Kuraman. Eds. Westview Press. Pp. 139-156.
- Youdeowei, A. (2002). Integrated Pest Management Practices for the Production of Root and Tubers and Plantains. Integrated Pest ManagementExtension Guide 3. Ministry of Food and Agriculture (MOFA) Plant Protection and Regulatory Services Directorate (PPRSD), Ghana, with German Development Cooperation (GTZ). ISBN: 9988-0-1087-7.
