|
African armyworm (Spodoptera exempta) It is usually an occasional pest, but when outbreaks occur damage to millet can be devastating. The caterpillars eat the above-ground parts of the plants leaving only the base of the stem. |
|
|
What to do:
|
|
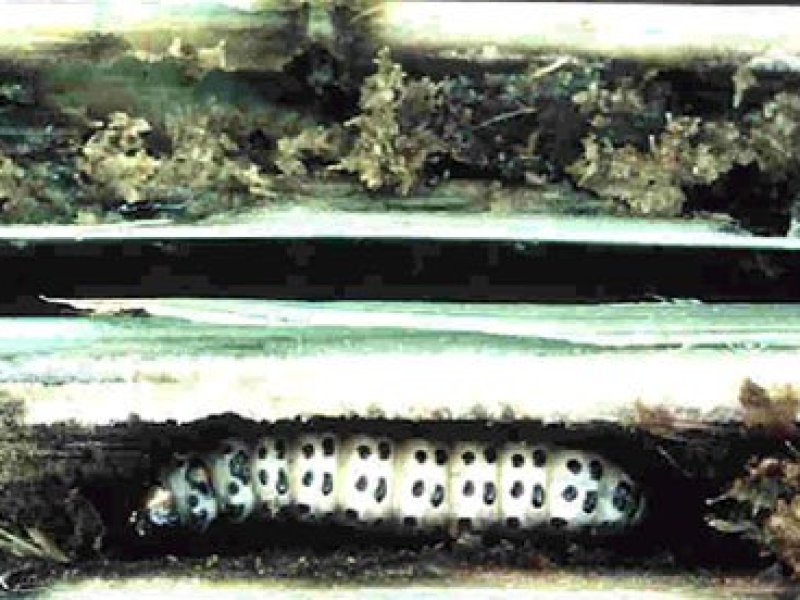
Stemborers: African maize stalkborer (Busseola fusca) Stemborers are the most important insect pests of maize in sub-Saharan Africa. Yield losses vary between 10-70%. Several species have been reported. The importance of a species varies between regions, within a country or even the same eco-region of neighbouring countries. At least four species attack maize in eastern and southern Africa, with yield losses reported to vary from 20 to 40%, depending on agroecological conditions, crop cultivars, agronomic practices and intensity of infestation.
The most important are the African maize stalkborer (Busseola fusca) and the spotted stemborer (Chilo partellus) (see also below).
The pink stalkborer (Sesamia calamistis) and the sugarcane stalkborer (Eldana saccharina) are of minor importance in maize.
Early warning signs: Young plants have pinholes in straight lines across the newest leaves. This is the time to treat - before the caterpillars move into the stem. |
|
|
What to do:
|
|
Blast (Pyricularia grisea) Lesions on foliage are elliptical or diamond-shaped, approximately 3 x 2 mm. Lesion centres are grey and water-soaked when fresh but turn brown upon drying. Lesions are often surrounded by a chlorotic halo, which will turn necrotic giving the appearance of concentric rings. The disease is favoured by hot, humid conditions.
|
|
|
What to do:
|
|
Crazy top downy mildew (Sclerospora graminicola) Symptoms often vary as a result of systemic infection. Leaf symptoms begin as chlorosis at the base and successively higher leaves show progressively greater chlorosis. On the lower leaf surface of infected leaves greyish white fungal growth may be observed. Severely infected plants are generally stunted and do not produce panicles. Green ear symptoms result from transformation of floral parts into leafy structures. The disease is prevalent during rainy seasons.
|
|
|
What to do:
|
|
Ergot (Claviceps spp.) Cream to pink sticky "honeydew" droplets ooze out of infected florets on panicles. Within 10 to 15 days, the droplets dry and harden, and dark brown to black sclerotia (fungal fruiting bodies) develop in place of seeds on the panicle. Sclerotia are larger than seed and irregularly shaped, and generally get mixed with the grain during threshing. Conditions favouring the disease are relative humidity greater than 80%, and temperatures between 20 to 30 degC. The sclerotia falling on the soil or planted with the seed germinate when the plants are flowering. They produce spores that are wind-borne to the flowers, where they invade the young kernels and replace the kernels with fungal growth. The fungal growth bears millions of tiny spores in a sticky, sweet, honeydew mass. These spores are carried by insects or splashed by rain to infect other kernels.
|
|
|
What to do:
|
|
Grasshoppers Several species of grasshoppers attack millets. Short-horned grasshoppers include Zonocerus spp, Oedaleus senegalensis, Kraussaria angulifera, Hieroglyphus daganensis, Diabolocantatops axillaris among others. The long horned edible grasshopper (Homorocoryphus niditulus) is a pest in East Africa. Grasshoppers defoliate and eat the panicles. They are not of economic importance when present in low numbers. However, invasion by a swarm of grasshoppers may result in serious grain losses.
|
|
|
What to do:
|
|
Long smut (Tolyposporium penicillariae) Immature, green fungal bodies (sori) larger than the seed develop on panicles during grain fill. A single fungal body (sorus) develops per floret. As grain matures, sori change in colour from green to dark brown. Sori are filled with dark spores. Infection takes place at temperature range of between 21 and 31°C, and at relative humidity greater than 80%. The disease is spread by wind-borne spores and rain.
|
|
|
What to do:
|
|
Mealybugs Mealybugs are small, flat, soft bodied insects covered with a distinctive segmentation. Their body is covered with a white woolly secretion. They suck sap from tender leaves, petioles and fruits. Seriously attacked leaves turn yellow and eventually dry. This can lead to shedding of leaves, inflorescences, and young fruit. Mealybugs excrete honeydew on which sooty mould developed. Heavy coating with honeydew blackens the leaves, branches and fruit. This reduces photosynthesis, can cause leaf drop and affect the market value of the fruit.
A wide range of natural enemies attacks mealybugs. The most important are ladybird beetles, hover flies, lacewings, and parasitic wasps. These natural enemies usually control mealybugs. However, mealybugs can cause economic damage to mango when natural enemies are disturbed (for instance by ants feeding on honeydew produced by mealybugs or other insects) or killed by broad-spectrum pesticides, or when mealybugs are introduced to new areas, where there are no efficient natural enemies.
The latter is the case of two serious mealybug pests on mangoes in Africa: Rastrococcus invadens in West and Central Africa and Rastrococcus iceryoides in East Africa. These mealybugs, of Asian origin, were introduced into Africa, where they developed into serious pests since the natural enemies present were not able to control them. They cause shedding of leaves, inflorescences and young fruits. In addition, sooty moulds growing on honeydews excreted by the insects render the fruits unmarketable and the trees unsuitable for shading. They cause direct damage to fruits leading to 40 to 80% losses depending on locality, variety and season. Rastrococcus invadens was brought under control in West and Central Africa by two parasitic wasps (Gyranusoidea tebygi and Anagyrus mangicola) introduced from India (Neuenschwander, 2003).
Rastrococcus iceryoides is a major pest of mango in East Africa, mainly Tanzania and coastal Kenya. Although several natural enemies are known to attack this mealybug in its aboriginal home of southern Asia (Tandon and Lal, 1978; CABI, 2000), none have been introduced so far into Africa (ICIPE). Insecticides do not generally provide adequate control of mealybugs owing to their wax coating. |
|
|
What to do:
|
|
Millet head miner (Heliocheilus albipunctella) It is the most important insect pest of pearl millet in the Sahel. Moths deposit their eggs on the heads of millet, preferring half-emerged and fully-emerged flowering heads. The caterpillars mine into the seeds of the millet head, damaging the millet panicle (i.e. the flower head, where the grain is formed). It has been reported to cause complete crop loss. Pupation takes place in the soil.
|
|
|
What to do:
|
|
Shoot fly (Atherigona soccata) Sorghum shoot fly, (Atherigona soccata), is a particularly nasty pest of sorghum in Asia, Africa, and the Mediterranean area. Females lay single cigar-shaped eggs on the undersides of leaves at the 1- to 7-leaf stage. The eggs hatch after only a day or two of incubation, and the larvae cut the growing point of the leaf, resulting in wilting and drying. These leaves, known as 'deadhearts', are easily plucked. When a "dead heart" is plucked, it releases an obnoxious odor. Adult resemble small houseflies. They are about 0.5 cm long. The shoot fly has been reported as attacking pearl millet. Damage occurs 1-4 weeks after seedling emergence. The damaged plants produce side tillers, which may also be attacked. The shoot fly's entire life cycle is completed in 17-21 days. Infestations are especially high when sorghum planting is staggered due to erratic rainfall. Temperatures above 35°C and below 18°C reduce shoot fly survival, as does continuous rainfall. |
|
|
What to do:
|
|
Stemborers Several species of stemborers attack millet including the millet stemborer (Coniesta ignefusalis), the maize stalkborer (Busseola fusca), the spotted stalkborer (Chilo partellus), and the pink stalkborer (Sesamia calamitis). Stemborer caterpillars bore into stems of millets disrupting the flow of nutrient from the roots to the upper parts of plants. Attack on young millet plants causes damage known as "dead hearts". In older plants the top part of the stem dies as a result of tunnelling by the borers.
The millet stemborer (Coniesta ignefusalis) It is the dominant stemborer of millet in the Sahelian zone of Africa, and also attacks sorghum, maize, and wild grasses. Major damage has been reported in West Africa. It has also been found causing considerable damage to millet in Western Eritrea, being considered as the major pest of millets in Eritrea (B. Le Ru, icipe, personal communication). The moths have golden brown forewings. They are active throughout the night and during the day rest on the lower surface of leaves or along stems. Caterpillars are cream-coloured with black spots along the body. However in the dry season, when caterpillars enter in diapause (a resting period) they change colour to pale yellow or uniform cream white. They stay in this resting period from 6 to 7 months, but occasionally for more than a year.
Moths lay eggs between the leaf sheet and the stem in batches of 20 to 50 eggs. Caterpillars tunnel in the leaf sheets and in the underlying stem. They normally pupate within the stem. Small plants on which eggs are laid may be thoroughly riddle with caterpillars and soon collapse, but in larger plants external symptoms show two to three weeks after stems have been infested. Economic damage results from early plant death ( "dead-heart") stem tunnelling, disruption of nutrient flow, steam breakage, poor or no grain formation and empty heads. Crop losses have been estimated at $91 million a year. For more information on the African maize stalk borer click here. For more information on the Spotted stalkborer click here. |
|
|
What to do:
|
|
Storage pests: The lesser grain borer (Rhyzopertha dominica) and the khapra beetle (Trogoderma granarium) Grains of pearl millet are attacked by major pests such as the lesser grain borer and the khapra beetle. For this reason, the popular concept that millets are hardly susceptible to damage by storage insect pests is erroneous, except for the very small-grained millets such as tef and fonio. The lesser grain borer and the kapra beetle are relatively well adapted to extremely dry conditions and will cause serious damage to millet. Other secondary storage pests do not thrive in semi-arid climates where millets are grown, where stored grain is typically very dry. Other non-insect pests such as rats and birds may destroy a considerable part of the harvest. |
|
|
What to do:
|
Geographical Distribution in Africa
Geographical Distribution of Millet in Africa. Updated 8th July 2019. Source FAOSTAT
General Information and Agronomic Aspects
Millets refers to a group of annual grasses mainly found in the arid and semiarid regions of the world. These grasses produce small seeded grains and are often cultivated as cereals. The most widely cultivated species are:
- Pearl millet (Pennisetum glaucum)
- Foxtail millet (Setaria italica)
- Common millet or proso millet (Panicum miliaceum)
- Finger millet (Eleusine coracana)
The husked grain of millet has a slightly nutty flavour and can be eaten whole after roasting or after cooking or boiling like rice. Millet flour is used for making mush, porridge, flat bread or chapatti. The flour is also used for making wine or beer. The grain is a feed for animals. The green plant is used as forage, but the quality of the straw is poor. Brooms are made from the straw. Starch from the grains is used for sizing textiles. Millet plays a vital role as a food security crop especially in semi arid lands of Kenya. Some millet varieties will survive drought conditions where maize crops often fail to reach maturity. The popularity of millet fell for some years due to introduction of maize, wheat and rice, but is again on the rise with millers being able to sell far more than is delivered. Millet is fast becoming a popular baby food as the grains are rich in calcium and have a pleasant flavour. Due to unpredictable rainfall patterns, Kenya has been experiencing frequent maize crop failure (the main staple) leading the Government of Kenya to encourage the production of indigenous, drought tolerant crops like millet.
Nutritive Value per 100 g of edible Portion
| Raw or Cooked Millet | Food Energy (Calories / %Daily Value*) |
Carbohydrates (g / %DV) |
Fat (g / %DV) |
Protein (g / %DV) |
Calcium (g / %DV) |
Phosphorus (mg / %DV) |
Iron (mg / %DV) |
Potassium (mg / %DV) |
Vitamin A (I.U) |
Vitamin C (I.U) |
Vitamin B 6 (I.U) |
Vitamin B 12 (I.U) |
Thiamine (mg / %DV) |
Riboflavin (mg / %DV) |
Ash (g / %DV) |
| Millet cooked | 119 / 6% | 23.7 / 8% | 1.0 / 2% | 3.5 / 7% | 3.0 / 0% | 100.0 / 10% | 0.6 / 3% | 62.0 / 2% | 3.0 IU / 0% | 0.0 / 0% | 0.1 / 5% | 0.0 / 0% | 0.1 / 7% | 0.1 / 5% | 0.4 |
| Millet puffed | 354 / 18% | 80.0 / 27% | 3.4 / 5% | 13.0 / 26% | 8.0 / 1% | 266 / 27% | 2.8 / 16% | 40.0 / 1% | 0.0 IU / 0% | 0.0 / 0% | 0.4 / 18% | 0.0 / 0% | 0.4 / 26% | 0.3 / 16% | 1.6 |
| Millet raw | 378 / 19% | 72.9 / 24% | 4.2 / 6% | 11.0 / 22% | 8.0 / 1% | 285 / 28% | 3.0 / 17% | 195 / 6% | 0.0 IU / 0% | 0.0 / 0% | 0.4 / 19% | 0.0 / 0% | 0.4 / 28% | 0.3 / 17% | 3.3 |
*Percent Daily Values (DV) are based on a 2000 calorie diet. Your daily values may be higher or lower, depending on your calorie needs.
Climate conditions, soil and water management
Millet is mostly grown in temperate and subtropical regions. It is adapted to conditions that are too hot and too dry, and to soils too shallow and poor for successful cultivation of other cereals. It is tolerant to a very wide temperature range but susceptible to frost. Cultivation occurs up to 3000 m altitude in the Himalayas. In Kenya millet is grown from 0 - 2400 m above sea level. Proso millet has one of the lowest water requirements of all cereals. An average annual rainfall of 200 - 450 mm is sufficient, of which 35 - 40% should fall during the growing period. Most soils are suitable for its cultivation, except coarse sand.
Varieties in Kenya
A lot of work has been done to identify improved varieties of millet to be grown under different ecological zones of Kenya.
Some recommended varieties of finger millets and their characteristics (Kenya)
 |
| Finger Millet |
|
© A.A.Seif, icipe
|
| Variety | Optimal production altitude (masl) |
Maturity (Months) |
Grain colour | Potential grain yield (90 kg bags/acre) |
Special attributes |
| "P 224" | 1150-1750 | 3-4 | Brown | 10 | Tolerant to lodging and blast |
| "Gulu E" | 250-1500 | 4 | Brown | 8 | - |
| "KAT/FMI" | 250-1150 | 3 | Brown | 7 | Drought tolerant. Tolerant to blast. High in calcium |
| "Lanet/FM1" | 1750-2300 | 5-7 | Brown | 7 | Tolerant to cold and drought |
Examples of finger millet varieties in Uganda
- "PESE 1"
- "PESE 2"
- "SEREMI 1"
- "SEREMI 2"
- "SEREMI 3"
Some recommended varieties of pearl millets and their characteristics (Kenya)
 |
| Pearl Millet |
|
© A.A.Seif, icipe
|
| Variety | Optimal production altitude (masl) |
Maturity (Months) |
Grain colour | Potential grain yield (90 kg bags/acre) |
Special attributes |
| "KAT/PM1" | 250-1150 | 2-3 | Grey | 8 | Tolerant to bird damage, leaf blight and rust |
| "KAT/PM2" | 250-1150 | 2 | Grey | 7 | Tolerant to leaf blight and rust. Grain used at dough stage |
| "KAT/PM3" | 50-1500 | 2-3 | Grey | 10 | Tolerant to leaf blight and rust |
Examples of pearl millet varieties in Tanzania
- "Okoa" (Altitude recommended: 0-1300 m; grain yield: 2.0-2.5 t/ha; grain colour: grey; days to flowering: 87-92; resistant to Striga spp.; tolerant to ergot)
- "Shibe" (Altitude recommended: 0-1200 m; grain yield: 1.8-2.0 t/ha; grain colour: grey; days to flowering: 90-95; resistant to Striga spp.)
 |
| Proso Millet |
|
© A. A.Seif, icipe
|
| Crop | Variety | Optimal production altitude (masl) |
Maturity (Months) |
Grain colour | Potential grain yield (90 kg bags/acre) |
Special attributes |
| Proso millet | "KAT/PRO-1" | 0-2000 | 2.5 | Cream | 7 | Has ability to stop growing during severe water stress and to resume growth quickly when the stress is broken |
| Fox tail millet | "KAT/FOX-1" | 250-1500 | 3-4 | Cream | 8 | - |
Proso and fox tail millets can be grown in all areas whereas "Gulu E" does best on coast and moist mid altitude. KAT/FM1 is recommended for semi-arid lowlands and Lanet/FM1 for cold semi arid highlands.
Propagation and planting
Selection of healthy seeds, free from bird and insect damage and diseases, is important to produce vigorous seedlings that could fare well in case of attack by pests or diseases. Prepare seed for sowing by threshing it (if at all stored on the head) and removing all mixtures such as glumes, bits of the rachis and peduncle, etc. This can be done by winnowing and occasionally by sieving. These processes also remove light and small seeds. A fast, easy and efficient method of quality seed selection uses a 10% salt solution to separate good seeds from bad seeds. The salt solution enhances the flotation of light and damaged seeds, fungal spores and light foreign matter. The good and heavy seeds and pebbles drop to the bottom. The floating portion is decanted and discarded and the sunken portion subjected to flotation one or two more times, after which the good seeds at the bottom are rinsed with clean water to remove excess salt. This portion is then sun-dried. After drying, the pebbles are removed by hand picking (DFPV, Niger).
Early land preparation is recommended. Millet requires a fine seedbed suitable for small grains, to ensure good germination, plant population density and effective weed control. If tractors or oxen are used to open up land for planting, it is advisable to harrow it after the first ploughing. When jembes (hand hoes) are used for land preparation, farmers are advised tobreak large clods to provide a smooth seedbed. Plant before or at the onset of rains by either drilling in the furrows made by oxen plough or tractor or by using a panga (cutlass) for hand planting in hills.
Spacing and seed rate
If the population is too high at emergence, thin when plants are about 15 cm tall, 2 weeks after emergence. Seed rate (when planted in furrows):
- Finger millet - 3 kg/ha
- Pearl millet - 5 kg/ha
- Fox tail millet - 4 kg/ha
- Proso millet - 4 kg/ha
For sole cropping the following distances should be followed:
- Pearl millet varieties: 15 cm between seeds and 60 cm between rows
- Finger millet, foxtail and Proso millet: 10 cm between seeds and 30 cm between rows.
Husbandry
Millet benefits from intercropping with legumes such as green gram and cowpeas. It can also be rotated with legume crops to benefit from the soil improvement facilitated by these crops or intercropped with other non-cereal crops. Application of farmyard manure at 8-10 tons/ha is recommended in order to improve the soil organic matter content, moisture retention ability and soil structure. Phosphorous should be applied in the form of rock phosphate. Weeding should be done twice, first time 2-3 weeks after emergence and second weeding about two weeks later.
Harvesting
Harvest takes place 2-4 months after sowing, when the grain has a moisture content of 14-15%. Avoid delayed harvesting, as the seed shatters easily. If millet is harvested during the rainy season with high relative humidity, the grain must be dried to 14% moisture content. In households, millet is usually dried above the domestic fire. Millet is threshed immediately after harvest. The grain stores well for up to five years. Sometimes the grain is mixed with ash or slightly baked before storage. Because of its small size, the grain is barely susceptible to insect attack.
| African armyworm (Spodoptera exempta) It is usually an occasional pest, but when outbreaks occur damage to millet can be devastating. The caterpillars eat the above-ground parts of the plants leaving only the base of the stem. What to do:
|
| Several species of stemborers attack millet including the millet stemborer (Coniesta ignefusalis), the maize stalkborer (Busseola fusca), the spotted stalkborer (Chilo partellus), and the pink stalkborer (Sesamia calamitis). Stemborer caterpillars bore into stems of millets disrupting the flow of nutrient from the roots to the upper parts of plants. Attack on young millet plants causes damage known as "dead hearts". In older plants the top part of the stem dies as a result of tunnelling by the borers.
The millet stemborer (Coniesta ignefusalis) It is the dominant stemborer of millet in the Sahelian zone of Africa, and also attacks sorghum, maize, and wild grasses. Major damage has been reported in West Africa. It has also been found causing considerable damage to millet in Western Eritrea, being considered as the major pest of millets in Eritrea (B. Le Ru, icipe, personal communication). The moths have golden brown forewings. They are active throughout the night and during the day rest on the lower surface of leaves or along stems. Caterpillars are cream-coloured with black spots along the body. However in the dry season, when caterpillars enter in diapause (a resting period) they change colour to pale yellow or uniform cream white. They stay in this resting period from 6 to 7 months, but occasionally for more than a year.
Moths lay eggs between the leaf sheet and the stem in batches of 20 to 50 eggs. Caterpillars tunnel in the leaf sheets and in the underlying stem. They normally pupate within the stem. Small plants on which eggs are laid may be thoroughly riddle with caterpillars and soon collapse, but in larger plants external symptoms show two to three weeks after stems have been infested. Economic damage results from early plant death ( "dead-heart") stem tunnelling, disruption of nutrient flow, steam breakage, poor or no grain formation and empty heads. Crop losses have been estimated at $91 million a year. For more information on the African maize stalk borer click here. For more information on the Spotted stalkborer click here. What to do:
|
| Millet head miner (Heliocheilus albipunctella) It is the most important insect pest of pearl millet in the Sahel. Moths deposit their eggs on the heads of millet, preferring half-emerged and fully-emerged flowering heads. The caterpillars mine into the seeds of the millet head, damaging the millet panicle (i.e. the flower head, where the grain is formed). It has been reported to cause complete crop loss. Pupation takes place in the soil.
What to do:
|
| Shoot fly (Atherigona soccata) Sorghum shoot fly, (Atherigona soccata), is a particularly nasty pest of sorghum in Asia, Africa, and the Mediterranean area. Females lay single cigar-shaped eggs on the undersides of leaves at the 1- to 7-leaf stage. The eggs hatch after only a day or two of incubation, and the larvae cut the growing point of the leaf, resulting in wilting and drying. These leaves, known as 'deadhearts', are easily plucked. When a "dead heart" is plucked, it releases an obnoxious odor. Adult resemble small houseflies. They are about 0.5 cm long. The shoot fly has been reported as attacking pearl millet. Damage occurs 1-4 weeks after seedling emergence. The damaged plants produce side tillers, which may also be attacked. The shoot fly's entire life cycle is completed in 17-21 days. Infestations are especially high when sorghum planting is staggered due to erratic rainfall. Temperatures above 35°C and below 18°C reduce shoot fly survival, as does continuous rainfall. What to do:
|
| Several species of grasshoppers attack millets. Short-horned grasshoppers include Zonocerus spp, Oedaleus senegalensis, Kraussaria angulifera, Hieroglyphus daganensis, Diabolocantatops axillaris among others. The long horned edible grasshopper (Homorocoryphus niditulus) is a pest in East Africa. Grasshoppers defoliate and eat the panicles. They are not of economic importance when present in low numbers. However, invasion by a swarm of grasshoppers may result in serious grain losses.
What to do:
|
| Grains of pearl millet are attacked by major pests such as the lesser grain borer and the khapra beetle. For this reason, the popular concept that millets are hardly susceptible to damage by storage insect pests is erroneous, except for the very small-grained millets such as tef and fonio. The lesser grain borer and the kapra beetle are relatively well adapted to extremely dry conditions and will cause serious damage to millet. Other secondary storage pests do not thrive in semi-arid climates where millets are grown, where stored grain is typically very dry. Other non-insect pests such as rats and birds may destroy a considerable part of the harvest. What to do:
|
| Stemborers: African maize stalkborer (Busseola fusca) Stemborers are the most important insect pests of maize in sub-Saharan Africa. Yield losses vary between 10-70%. Several species have been reported. The importance of a species varies between regions, within a country or even the same eco-region of neighbouring countries. At least four species attack maize in eastern and southern Africa, with yield losses reported to vary from 20 to 40%, depending on agroecological conditions, crop cultivars, agronomic practices and intensity of infestation.
The most important are the African maize stalkborer (Busseola fusca) and the spotted stemborer (Chilo partellus) (see also below).
The pink stalkborer (Sesamia calamistis) and the sugarcane stalkborer (Eldana saccharina) are of minor importance in maize.
Early warning signs: Young plants have pinholes in straight lines across the newest leaves. This is the time to treat - before the caterpillars move into the stem. What to do:
|
| Mealybugs are small, flat, soft bodied insects covered with a distinctive segmentation. Their body is covered with a white woolly secretion. They suck sap from tender leaves, petioles and fruits. Seriously attacked leaves turn yellow and eventually dry. This can lead to shedding of leaves, inflorescences, and young fruit. Mealybugs excrete honeydew on which sooty mould developed. Heavy coating with honeydew blackens the leaves, branches and fruit. This reduces photosynthesis, can cause leaf drop and affect the market value of the fruit.
A wide range of natural enemies attacks mealybugs. The most important are ladybird beetles, hover flies, lacewings, and parasitic wasps. These natural enemies usually control mealybugs. However, mealybugs can cause economic damage to mango when natural enemies are disturbed (for instance by ants feeding on honeydew produced by mealybugs or other insects) or killed by broad-spectrum pesticides, or when mealybugs are introduced to new areas, where there are no efficient natural enemies.
The latter is the case of two serious mealybug pests on mangoes in Africa: Rastrococcus invadens in West and Central Africa and Rastrococcus iceryoides in East Africa. These mealybugs, of Asian origin, were introduced into Africa, where they developed into serious pests since the natural enemies present were not able to control them. They cause shedding of leaves, inflorescences and young fruits. In addition, sooty moulds growing on honeydews excreted by the insects render the fruits unmarketable and the trees unsuitable for shading. They cause direct damage to fruits leading to 40 to 80% losses depending on locality, variety and season. Rastrococcus invadens was brought under control in West and Central Africa by two parasitic wasps (Gyranusoidea tebygi and Anagyrus mangicola) introduced from India (Neuenschwander, 2003).
Rastrococcus iceryoides is a major pest of mango in East Africa, mainly Tanzania and coastal Kenya. Although several natural enemies are known to attack this mealybug in its aboriginal home of southern Asia (Tandon and Lal, 1978; CABI, 2000), none have been introduced so far into Africa (ICIPE). Insecticides do not generally provide adequate control of mealybugs owing to their wax coating. What to do:
|
| Cream to pink sticky "honeydew" droplets ooze out of infected florets on panicles. Within 10 to 15 days, the droplets dry and harden, and dark brown to black sclerotia (fungal fruiting bodies) develop in place of seeds on the panicle. Sclerotia are larger than seed and irregularly shaped, and generally get mixed with the grain during threshing. Conditions favouring the disease are relative humidity greater than 80%, and temperatures between 20 to 30 degC. The sclerotia falling on the soil or planted with the seed germinate when the plants are flowering. They produce spores that are wind-borne to the flowers, where they invade the young kernels and replace the kernels with fungal growth. The fungal growth bears millions of tiny spores in a sticky, sweet, honeydew mass. These spores are carried by insects or splashed by rain to infect other kernels.
What to do:
|
| Lesions on foliage are elliptical or diamond-shaped, approximately 3 x 2 mm. Lesion centres are grey and water-soaked when fresh but turn brown upon drying. Lesions are often surrounded by a chlorotic halo, which will turn necrotic giving the appearance of concentric rings. The disease is favoured by hot, humid conditions.
What to do:
|
| Long smut (Tolyposporium penicillariae) Immature, green fungal bodies (sori) larger than the seed develop on panicles during grain fill. A single fungal body (sorus) develops per floret. As grain matures, sori change in colour from green to dark brown. Sori are filled with dark spores. Infection takes place at temperature range of between 21 and 31°C, and at relative humidity greater than 80%. The disease is spread by wind-borne spores and rain.
What to do:
|
| Crazy top downy mildew (Sclerospora graminicola) Symptoms often vary as a result of systemic infection. Leaf symptoms begin as chlorosis at the base and successively higher leaves show progressively greater chlorosis. On the lower leaf surface of infected leaves greyish white fungal growth may be observed. Severely infected plants are generally stunted and do not produce panicles. Green ear symptoms result from transformation of floral parts into leafy structures. The disease is prevalent during rainy seasons.
What to do:
|
Information Source Links
- AIC (2002). Field Crops Technical Handbook.
- ARS Agricultural Handbook No. 716: Diseases of Pearl Millet. www.tifton.uga.edu
- CAB International (2005). Crop Protection Compendium, 2005 edition. Wallingford, UK www.cabi.org
- Harris, K. M. (1962). Lepidopterous stem borers of cereals in Nigeria. Vol 53. 139-171
- ICRISAT. Millet production growing in Africa. designs improved cropping systems for Sahelian millet, cowpeas. www.worldbank.org
- Integrated management project of the millet head miner for increasing millet production in the Sahelian zone. www.ccrp.org
- INPhO, Post harvest compendium. Millet. www.fao.org
- Maymoona, A.E., Roth, M. (2007). Utilisation of diversitcharacteristics of the millet worm Heliocheilus albipunctella (Lepidoptera: noctuidae) a pest on millet in Sudan. Tropentag, October 9-11, 2007, Witzenhausen. www.tropentag.de
- National Research Council. 1996. Lost Crops of Africa: Volume I: Grains. Washington, DC: The National Academies Press. Available online: www.nap.edu
- Nutrition Data www.nutritiondata.com.
- Oduro, K.A. (2000). Checklist of Plant Pests in Ghana. Volume 1: Diseases. Ministry of Food and Agriculture. Plant Protection and Regulatory Services Directorate.
- Pheromone-based monitoring system to manage the millet stem borer Coniesta ignefusalis(Lepidoptera: Pyralidae). ICRISAT. www.icrisat.org
- Youdeowei, A. (2002). Integrated Pest Management Practices for the Production of Cereals and Pulses. Integrated Pest Management Extension Guide 2. Ministry of Food and Agriculture (MOFA) Plant Protection and Regulatory Services Directorate (PPRSD), Ghana, with German Development Cooperation (GTZ). ISBN: 9988 0 1086 9.
- Youm, O., Harris, K. M., Nwanze, K. F. (1996). Coniesta ignefusalis (Hampson), the millet stem borer: a handbook of information. Information Bulletin, no 46. Patancheru 502324, Andhra Pradesh, India: International Crops Research Institute for the Semi-Arid Tropics. 60 pp. ISBN 92-9066-253-0.